NCERT Solutions for Class 10 Science Chapter 1 Chemical Reactions and Equations includes answers of intext & exercise questions. All these NCERT solutions are prepared by expert teachers with detailed explanations of every important topic. It is important for the students to go through these NCERT solutions to get knowledge of the type of questions asked in Chemical reactions and equations chapter.
NCERT Solutions for Class 10 Science Chapter 1 Intext Questions
Page Number: 6
Question 1: Why should a magnesium ribbon be cleaned before burning in air?
Answer: Magnesium is a very reactive metal like (Na, Ca, etc.). When it is exposed to air it reacts with oxygen to form a layer of magnesium oxide (MgO) on its surface. This layer of magnesium oxide is quite stable and prevents further reaction of magnesium with oxygen. The magnesium ribbon is cleaned with sandpaper to remove this layer so that the underlying metal can be used for the reaction.
The Reaction involved in this case is as follows:
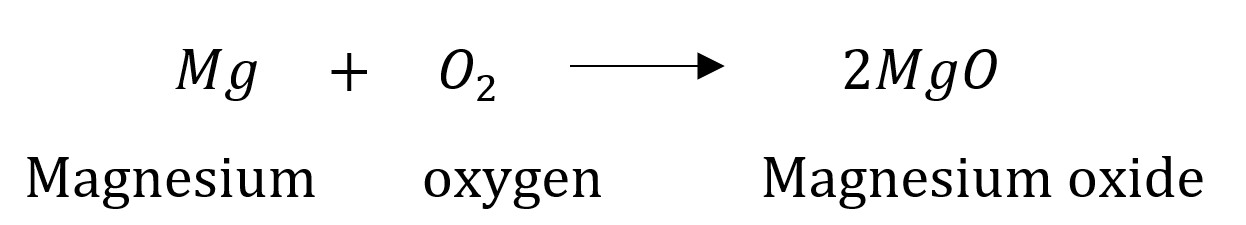
Question 2: Write the balanced equations for the following chemical reactions.
i) Hydrogen + Chloride → Hydrogen chloride
ii) Barium chloride + Aluminium sulphate → Barium sulphate + Aluminium chloride
iii) Sodium + Water → Sodium hydroxide + Hydrogen
Answer:
i) H2 + Cl2 → 2HCl
ii) 3BaCl2 + Al2(SO4)3 → 2AlCl3 + 3BaSO4
iii) 2Na + 2H2O → 2NaOH + H2
Question 3: Write a balanced chemical equation with state symbols for the following reactions.
(i) Solutions of barium chloride and sodium sulphate in water react to give insoluble barium sulphate and the solution of sodium chloride.
(ii) Sodium hydroxide solution (in water) reacts with hydrochloric acid solution (in water) to produce sodium chloride solution and water.
Answer:
(i) BaCl2 + Na2SO4 → BaSO4 + 2NaCl
(ii) NaOH + HCl → NaCl + H2O
Page Number: 10
Question 1: A solution of a substance ‘X’ is used for white washing.
(i) Name the substance ‘X’ and write its formula.
(ii) Write the reaction of the substance ‘X’ named in (i) above with water.
Answer:
(i) The substance ‘X’ is calcium oxide. Its chemical formula is CaO.
(ii) Calcium oxide reacts vigorously with water to form calcium hydroxide (slaked lime)
CaO + H2O → Ca(OH)2
Question 2: Why is the amount of gas collected in one of the test tubes in Activity 1.7 double of the amount collected in the other? Name this gas.
Answer: Using the Electrolysis of water, hydrogen and oxygen are get separated by the electricity. Water (H2O) contains two parts hydrogen and one parts oxygen. Since hydrogen goes to one test tube and oxygen goes to another, the amount of gas collected in one of the test tubes is double of the amount collected in the other.
Page Number: 13
Question 1: Why does the colour of copper sulphate solution change when an iron nail is dipped in it?
Answer: When an iron nail is dipped into a copper sulfate solution, the colour of the solution changes due to a chemical reaction known as a displacement reaction. In this reaction, iron (which is more reactive than copper) displaces copper from copper sulphate solution forming iron sulphate, which is green in colour. Therefore, the blue colour of copper sulphate solution fades and green colour appears.
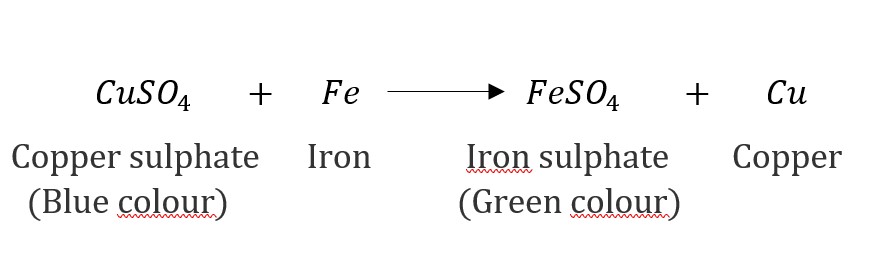
Question 2: Give an example of a double displacement reaction other than the one given in Activity 1.10.
Answer: Sodium carbonate reacts with calcium chloride to form calcium carbonate and sodium chloride.
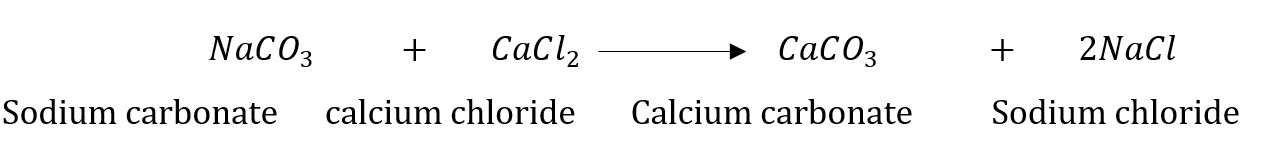
In this reaction, sodium carbonate and calcium chloride exchange ions to form two new compounds. Hence, it is a double displacement reaction.
Question 3: Identify the substances that are oxidised and the substances that are reduced in the following reactions.
(i) 4Na(s) + O2(g) → 2Na2O(s)
(ii) CuO(s) + H2(g) → Cu(s) + H2O(l)
Answer: (i) Sodium (Na) is oxidized as it gains oxygen and oxygen gets reduced.
(ii) Copper oxide (CuO) is reduced to copper (Cu) while hydrogen (H2) gets oxidized to water (H2O)
NCERT Solutions for Class 10 Science Chapter 1 Exercise Questions
Question 1: Which of the statements about the reaction below are incorrect?
2PbO(s) + C(s) → 2Pb(s) + CO2(g)
(a) Lead is getting reduced
(b) Carbon Dioxide is getting oxidised
(c) Carbon is getting oxidised
(d) Lead oxide is getting reduced
(i) (a) and (b)
(ii) (a) and (c)
(iii) (a), (b) and (c)
(iv) all
Answer: (i) (a) and (b)
Explanation: (a) because Oxygen is being removed and (b) because the removed oxygen from Lead is added to the elemental Carbon.
Question 2: Fe2O3 + 2Al → Al2O3 + 2Fe
The above reaction is an example of a
(a) combination reaction
(b) double displacement reaction
(c) decomposition reaction
(d) displacement reaction
Answer: (d) Displacement reaction.
Question 3: What happens when dilute hydrochloric acid is added to iron filings? Tick the correct answer.
(a) Hydrogen gas and iron chloride are produced.
(b) Chlorine gas and iron hydroxide are produced.
(c) No reaction takes place.
(d) Iron salt and water are produced.
Answer: (a) Hydrogen gas and iron chloride are produced.
Question 4: What is a balanced chemical equation? Why should chemical equations be balanced?
Answer: A balanced chemical equation is a chemical equation in which the number of atoms for each element in the reactants side is equal to the number of atoms of that element in the products side.
This balance is necessary because it reflects the law of conservation of mass, which states that mass cannot be created or destroyed in an ordinary chemical reaction. It means that the total number of atoms of each element should be equal on both sides of a chemical equation. Hence, chemical equations should be balanced.
Question 5: Translate the following statements into chemical equations and then balance them.
(a) Hydrogen gas combines with nitrogen to form ammonia.
(b) Hydrogen sulphide gas burns in air to give water and sulphur dioxide.
(c) Barium chloride reacts with aluminium sulphate to give aluminium chloride and a precipitate of barium sulphate.
(d) Potassium metal reacts with water to give potassium hydroxide and hydrogen gas.
Answer:
(a) 3H2 (g) + N2 (g) → 2NH3 (g)
(b) H2S (g) + 3O2 (g) → SO2 (g) + 2H2O(l)
(c) 3BaCl2 (aq) + Al2(SO4)3 (aq) → 2AlCl3 (aq) + 3BaSO4 ↓(s)
(d) 2K (s) + 2H2O (l) → 2KOH (aq) + H2 (g)
Question 6: Balance the following chemical equations :
(a) HNO3 + Ca (OH)2 → Ca (NO3)2 + H2O
(b) NaOH + H2SO4 → Na2SO4 + H2O
(c) NaCl + AgNO3 → AgCl + NaNO3
(d) BaCl2 + H2SO4 → BaSO4 + HCl
Answer:
(a) 2HNO3 + Ca(OH)2 → Ca(NO3)2 + 2H2O
(b) 2NaOH + H2SO4 → Na2SO4 + 2H2O
(c) NaCl + AgNO3 → AgCl + NaNO3
(d) BaCl2 + H2SO4 → BaSO4 + 2HCl
Question 7: Write the balanced chemical equations for the following reactions :
(a) Calcium hydroxide + Carbon dioxide → Calcium carbonate + Water
(b) Zinc + Silver nitrate → Zinc nitrate + Silver
(c) Aluminium + Copper chloride → Aluminium chloride + Copper
(d) Barium chloride + Potassium sulphate → Barium sulphate + Potassium chloride
Answer:
(a) Ca (OH)2 + CO2 → CaCO3 + H2O
(b) Zn + 2AgNO3 → Zn(NO3)2 + 2Ag
(c) 2Al + 3 CuCl2 → 2AlCl3 + 3Cu
(d) BaCl2 + K2SO4 → BaSO4 + 2KCl
Question 8: Write the balanced chemical equation for the following and identify the type of reaction in each case :
(a) Potassium bromide (aq) + Barium iodide (aq) → Potassium iodide (aq) + Barium
(b) Zinc carbonate(s) → Zinc oxide (s) + Carbon dioxide (g) bromide(s)
(c) Hydrogen (g) + Chloride (g) → Hydrogen chloride (g)
(d) Magnesium (s) + Hydrochloric acid (aq) → Magnesium chloride (aq) + Hydrogen (g)
Answer:
(a) 2KBr (aq) + Bal2(aq) → 2Kl(aq) + BaBr2(s)
Type: Double displacement reaction
(b) ZnCO3 (s) → ZnO (s) + CO2 (g)
Type: Decomposition reaction
(c) H2 (g) + Cl2 (g) → 2HCl(g)
Type: Combination reaction
(d) Mg (s) + 2HCl (aq) → MgCl2 (aq) + H2 (g)
Type: Displacement reaction
Question 9: What does one mean by exothermic and endothermic reactions? Give examples.
Answer: Exothermic reactions: Chemical reactions that release energy in the form of heat, light, or sound are called exothermic reactions.
Example: Mixture of sodium and chlorine to yield table salt.
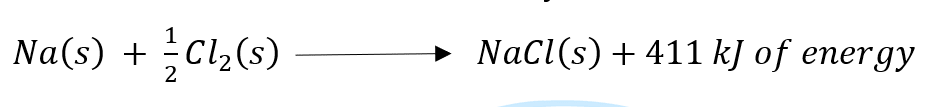
In other words, combination reactions are exothermic.
Endothermic reactions: Reactions that absorb energy or require energy to proceed are called endothermic reactions.
For example: In the process of photosynthesis, plants use the energy from the sun to convert carbon dioxide and water to glucose and oxygen.

Question 10: Why is respiration considered an exothermic reaction? Explain.
Answer: Respiration is considered an exothermic reaction because it releases energy, primarily in the form of heat, as it converts nutrients into usable energy. The energy in our body is obtained from the food we eat. During digestion, large molecules of food are broken down into simpler substances such as glucose. Glucose combines with oxygen in the cells and provides energy. The special name of this combustion reaction is respiration. Since energy is released in the whole process, it is an exothermic process.
In aerobic respiration, oxygen is consumed and carbon dioxide is produced along with water. The breakdown of glucose in the presence of oxygen (aerobic respiration) can be summarized by the equation:

Question 11: Why are decomposition reactions called the opposite of combination reactions? Write equations for these reactions.
Answer: Decomposition reactions are considered the opposite of combination reactions because, in decomposition, a complex molecule breaks down into simpler parts, while in a combination reaction, simple molecules combine to form a more complex molecule.
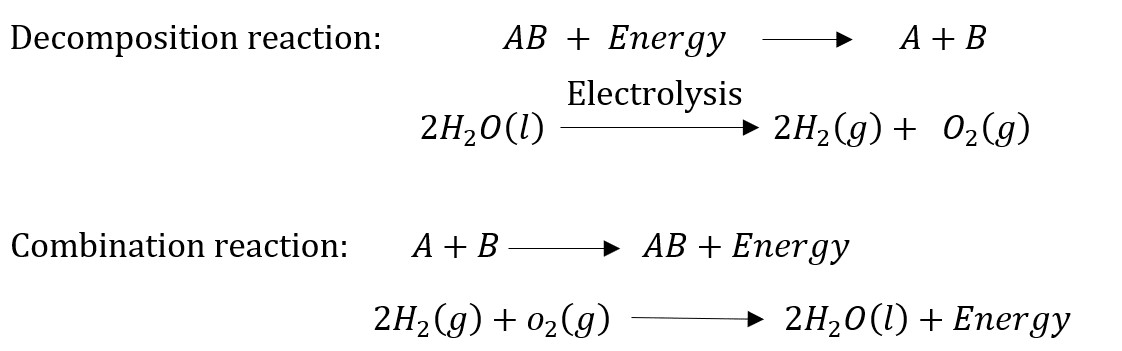
Question 12: Write one equation each for decomposition reactions where energy is supplied in the form of heat, light or electricity.
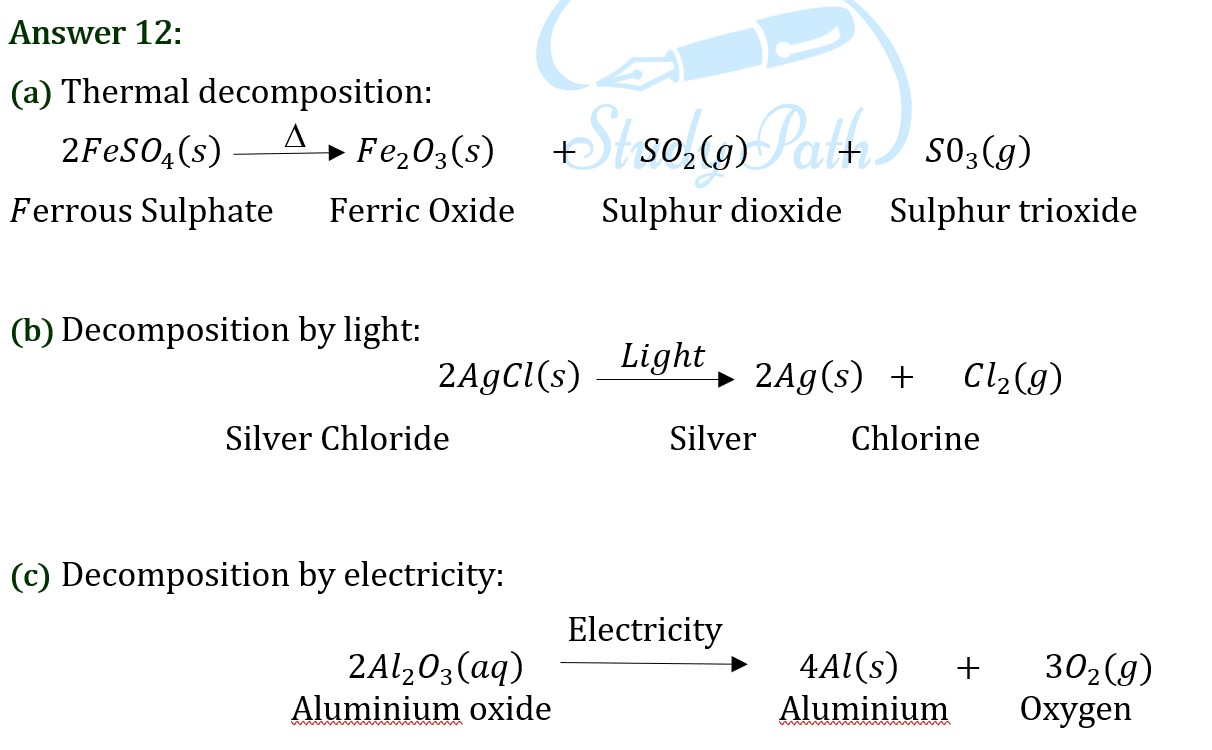
Question 13: What is the difference between displacement and double displacement reactions? Write equations for these reactions.
Answer: In a displacement reaction, a more reactive element replaces a less reactive element from a compound. For example:
Fe(s) + CuSO4(aq) → Cu(s) + FeSO4(aq)
This is a displacement reaction where iron displaces copper from its solution.
In a double displacement reaction, two atoms or a group of atoms switch places to form new compounds. For example:
AgNO3(aq) + NaCl (aq) → AgCl(s) + NaNO3 (aq)
This is a double displacement reaction where silver nitrate and sodium chloride exchange Cl– and NO3–ions between them.
Question 14: In the refining of silver, the recovery of silver from silver nitrate solution involved displacement by copper metal. Write down the reaction involved.
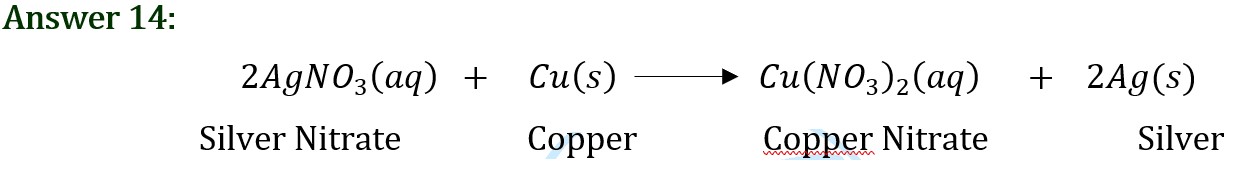
Question 15: What do you mean by a precipitation reaction? Explain by giving examples.
Answer: A reaction in which an insoluble solid (called precipitate) is formed is called a precipitation reaction. For example:
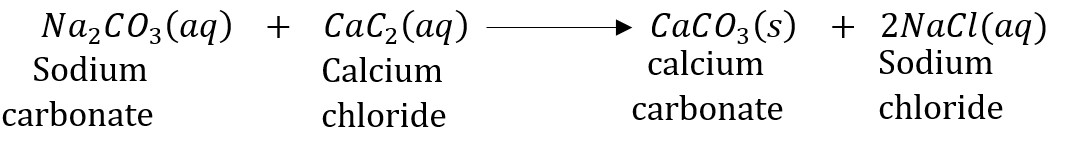
In this reaction, calcium carbonate is obtained as a precipitate. Hence, it is a precipitation reaction.
Another example of precipitation reaction is:
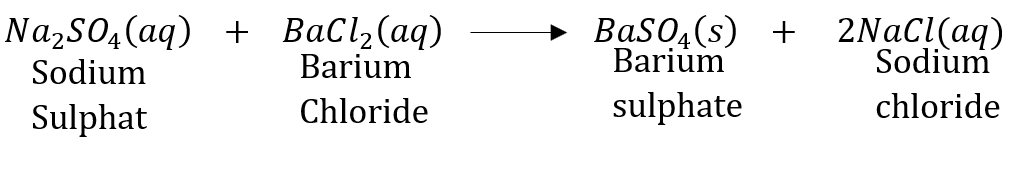
In this reaction, barium sulphate is obtained as a precipitate.
Question 16: Explain the following in terms of gain or loss of oxygen with two examples each:
(a) Oxidation and
(b) Reduction.
Answer:
(a) In a chemical reaction, when the oxygen is added to the element to form its respective oxide it is the element being oxidised. Example:
4Na(s) + O2(g) → 2Na2O(s)
H2S + O2 → H2O + SO2
(b) In a chemical reaction, when the oxygen is being removed from the compound then it is said to be reduced. Example:
CuO(s) + H2(g) → Cu(s) + H2O(l)
2HgO → 2Hg + O2
Question 17: A shiny brown-coloured element ‘X’ on heating in air becomes black in colour. Name the element ‘X’ and the black coloured compound formed.
Answer: ‘X’ is copper (Cu) and the black-coloured compound formed is copper oxide (CuO).
The equation of the reaction involved in heating copper is given below.
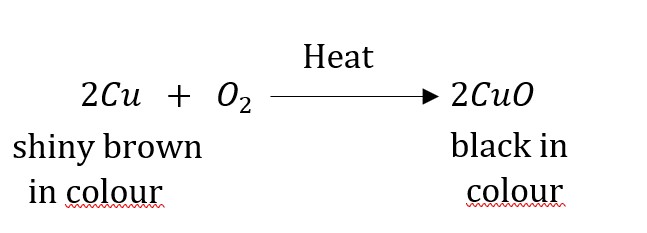
Question 18: Why do we apply paint on iron articles?
Answer: Iron articles are painted because it prevents them from rusting. When painted, the contact of iron articles from moisture and air is cut off. Hence, rusting is prevented their presence is essential for rusting to take place.
Question 19: Oil and fat containing food items are flushed with nitrogen. Why?
Answer: Nitrogen is an inert gas and does not easily react with these substances. On the other hand, oxygen reacts with food substances and makes them rancid. Thus, bags used in packing food items are flushed with nitrogen gas to remove oxygen inside the pack. When oxygen is not present inside the pack, rancidity of oil and fat containing food items is avoided.
Question 20: Explain the following terms with one example each.
(a) Corrosion
(b) Rancidity
Answer:
(a) Corrosion: Corrosion is defined as a process where materials, usually metals, deteriorate as a result of a chemical reaction with air, moisture, chemicals, etc. For example, iron, in the presence of moisture, reacts with oxygen to form hydrated iron oxide.
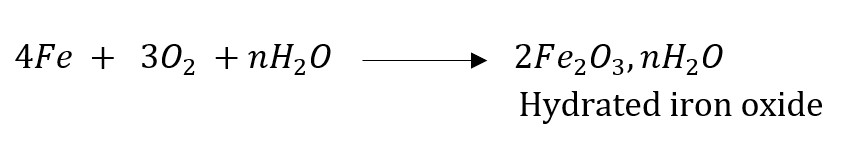
This hydrated iron oxide is rust.
(b) Rancidity: The process of oxidation of fats and oils that can be easily noticed by the change in taste and smell is known as rancidity.
For example, the taste and smell of butter changes when kept for long. Rancidity can be avoided by:
- Storing food in airtight containers
- Storing food in refrigerators
- Adding antioxidants
- Storing food in an environment of nitrogen